An ultrathin cobalt–iron oxide catalyst for water oxidation on nanostructured hematite photoanodes†
Abstract
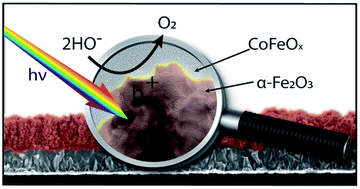
The harvesting of sunlight by a photoelectrochemical (PEC) cell to split water into hydrogen and oxygen is an attractive strategy to store solar energy in the form of chemical bonds. The oxygen evolution reaction (OER) remains a bottleneck for the development of efficient PEC devices. Here we report a photoelectrochemical method to homogeneously deposit a cobalt–iron oxide (CoFeOx) catalyst on a nanostructured hematite photoanode. An ultrathin catalyst layer (<1 nm) yielded a 200 mV cathodic shift of onset potential and a photocurrent density of 1.6 and 2.5 mA cm−2 at 1.0 V and 1.23 vs. RHE in 1 M KOH, respectively. We investigated the enhancement of photoactivity induced by the addition of the CoFeOx layer by impedance spectroscopy, photoluminescence, and by using H2O2 as a hole scavenger. This work points to the effective utilization of subnanometric coatings as efficient catalyst overlayers to enhance the OER activity of photoanodes.


 Please wait while we load your content...
Please wait while we load your content...