Surface reactivity and cation non-stoichiometry in BaZr1−xYxO3−δ (x = 0–0.2) exposed to CO2 at elevated temperature†
Abstract
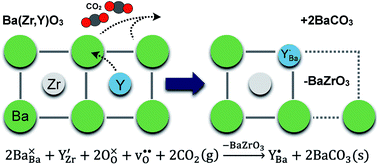
The reactivity of BaZr1−xYxO3−δ (x = 0–0.2) ceramics under 1 atm CO2 at 650 °C for up to 1000 h was investigated in order to elucidate possible degradation processes occurring when the material is applied as a proton-conducting electrolyte in electrochemical devices. The annealed ceramics were characterized by a range of techniques (SEM, TEM, GIXRD, XPS and SIMS) with respect to changes in the phase composition and microstructure. Formation of BaCO3 was observed on the surfaces of the annealed samples and the amount increased with time and was higher for the Y-doped compositions. The subsurface regions were found to be deficient in Ba and, in the case of the Y-doped compositions, enriched in Y in two distinct chemical states as identified by XPS. First-principles calculations showed that they were Y residing on the Zr and Ba-sites, respectively, and that local enrichment of Y both in bulk and on the surface attained a structure similar to Y2O3. Overall, it was substantiated that the reaction with CO2 mainly proceeded according to a defect chemical reaction involving transfer of Y to the Ba-site and consumption of BaZrO3 formula units. It was suggested that a similar degradation mechanism may occur in the case of Ba(OH)2 formation under high steam pressure conditions.


 Please wait while we load your content...
Please wait while we load your content...