Na3V2(PO4)2F3–SWCNT: a high voltage cathode for non-aqueous and aqueous sodium-ion batteries†
Abstract
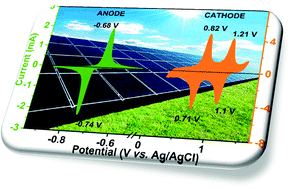
Due to the merits of low cost, safety, environmental friendliness, and abundant sodium reserves, non-aqueous and aqueous sodium-ion batteries are wonderful alternatives for large-scale energy storage. Na+ super ionic conductor structured Na3V2(PO4)2F3 is considered as a potential high-capacity cathode material for Na-ion batteries. However, its insufficient cyclability remains a challenge for battery applications. In addition, the narrow electrochemical stability window (1.23 V) of aqueous electrolytes does not allow us to make the best use of it. All of these pose obstacles to the practical voltage and energy output. Therefore, we designed and synthesized a Na3V2(PO4)2F3–SWCNT composite, which demonstrated superior Na+-storage performance with a high reversible capacity of 117 mA h g−1 at a moderate current of 0.5C in non-aqueous electrolyte. In addition, we developed an aqueous rechargeable sodium ion battery using Na3V2(PO4)2F3–SWCNT as the cathode and NaTi2(PO4)3–MWCNT as the anode, and this battery can deliver a high energy density of 150 W h kg−1 with an operating voltage of up to 2.0 V by using the high concentration electrolyte, 17 m NaClO4. The outstanding electrochemical performance makes Na3V2(PO4)2F3–SWCNT a promising cathode for Na+ storage and encourages more investigations into practical sodium-ion battery applications.


 Please wait while we load your content...
Please wait while we load your content...