Oxygen loss and surface degradation during electrochemical cycling of lithium-ion battery cathode material LiMn2O4†
Abstract
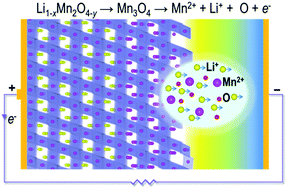
Electrode surfaces play a critical role in determining the electrochemical performance of lithium-ion batteries, and uncovering how surface chemistry and structure evolve during cycling, particularly at the atomic level, is necessary for improved battery materials design. We report a scanning transmission electron microscopy (STEM) investigation into the atomistic mechanisms behind surface reconstruction induced by electrochemical cycling of cathode material LiMn2O4. Direct STEM observations reveal that surface layers of as-synthesised LiMn2O4 thin films are subject to considerable compressive lattice strain as a result of oxygen deficiency. During the first charge, the lattice strain increases significantly, resulting in a reconstruction reaction to form Mn3O4 with further loss of oxygen from the topmost layers. Continued cycling leads to deterioration of surface crystallinity. The observed irreversible structure changes affect charge transfer reaction kinetics at LiMn2O4 surfaces because Li pathways become blocked by Mn atoms, contributing to a reduction in long-term cycle life and energy capacity. The ability to observe atomic-level changes at electrode surfaces at different stages of cycling provides a more robust understanding of electrode processes that can accelerate development of safer and longer-lasting battery materials.


 Please wait while we load your content...
Please wait while we load your content...