Amphiphilic microgels adsorbed at oil–water interfaces as mixers of two immiscible liquids†
Abstract
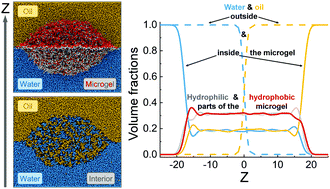
Amphiphilic microgels adsorbed at an oil–water interface were studied by means of dissipative particle dynamics (DPD) simulations. The hydrophobic (A) and hydrophilic (B) monomer units in the polymer network are considered to be randomly distributed. Effects of the crosslinking density, interfacial tension between the liquids, their selectivity as solvents towards species A and B, and the degree of incompatibility between the A and B units on the internal microgel structure and distribution of the liquids are considered. The most important predictions are that (i) two immiscible liquids can homogeneously be mixed within the microgels and (ii) the adsorbed microgels contain a high fraction of the liquids (they are swollen at the interface). Simultaneous fulfillment of these two conditions can have a high impact on the design of new and efficient catalytic systems. In particular, such microgels can mix immiscible reactants dissolved in water and oil and trigger chemical reactions in the presence of a catalyst embedded into the microgel.


 Please wait while we load your content...
Please wait while we load your content...