Controlling the strength of interaction between carbon dioxide and nitrogen-rich carbon materials by molecular design†
Abstract
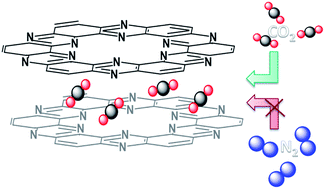
Thermal treatment of hexaazatriphenylene-hexacarbonitrile (HAT-CN) in the temperature range from 500 °C to 700 °C leads to precise control over the degree of condensation, and thus atomic construction and porosity of the resulting C2N-type materials. Depending on the condensation temperature of HAT-CN, nitrogen contents of more than 30 at% can be reached. In general, these carbons show adsorption properties which are comparable to those known for zeolites but their pore size can be adjusted over a wider range. At condensation temperatures of 525 °C and below, the uptake of nitrogen gas remains negligible due to size exclusion, but the internal pores are large and polarizing enough that CO2 can still adsorb on part of the internal surface. This leads to surprisingly high CO2 adsorption capacities and isosteric heat of adsorption of up to 52 kJ mol−1. Theoretical calculations show that this high binding enthalpy arises from collective stabilization effects from the nitrogen atoms in the C2N layers surrounding the carbon atom in the CO2 molecule and from the electron acceptor properties of the carbon atoms from C2N which are in close proximity to the oxygen atoms in CO2. A true CO2 molecular sieving effect is achieved for the first time in such a metal-free organic material with zeolite-like properties, showing an IAST CO2/N2 selectivity of up to 121 at 298 K and a N2/CO2 ratio of 90/10 without notable changes in the CO2 adsorption properities over 80 cycles.


 Please wait while we load your content...
Please wait while we load your content...