Evaluation of fluorine and sulfonic acid co-functionalized graphene oxide membranes under hydrogen proton exchange membrane fuel cell conditions†
Abstract
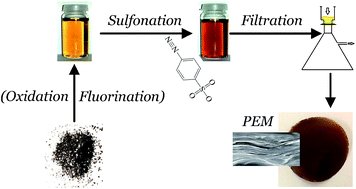
The use of graphene oxide (GO) based membranes consisting of self-assembled flakes with a lamellar structure represents an intriguing strategy to spatially separate reactants while facilitating proton transport in proton exchange membranes (PEMs). Here we chemically modify GO to evaluate the effect of fluorine and sulfonic acid groups on the performance of H2/O2 based PEM fuel cells. Mild fluorination is achieved in the presence of hydrogen fluoride during oxidation and subsequent sulfonation resulted in fluorine and SO3− co-functionalized GO. Membrane electrode assembly performance under low temperature and moderate humidity conditions suggested that both functional groups contribute to reduced H2 crossover compared to appropriate reference membranes. Moreover, fluorine groups promoted an enhanced hydrolytic stability while contributing to the prevention of structural degradation after constant potential experiments, whereas sulfonic acid exerted a stabilizing effect by preserving proton conductivity.


 Please wait while we load your content...
Please wait while we load your content...