Enzyme-mediated dynamic combinatorial chemistry allows out-of-equilibrium template-directed synthesis of macrocyclic oligosaccharides†
Abstract
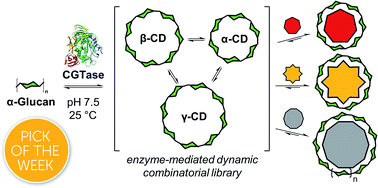
We show that the outcome of enzymatic reactions can be manipulated and controlled by using artificial template molecules to direct the self-assembly of specific products in an enzyme-mediated dynamic system. Specifically, we utilize a glycosyltransferase to generate a complex dynamic mixture of interconverting linear and macrocyclic α-1,4-D-glucans (cyclodextrins). We find that the native cyclodextrins (α, β and γ) are formed out-of-equilibrium as part of a kinetically trapped subsystem, that surprisingly operates transiently like a Dynamic Combinatorial Library (DCL) under thermodynamic control. By addition of different templates, we can promote the synthesis of each of the native cyclodextrins with 89–99% selectivity, or alternatively, we can amplify the synthesis of unusual large-ring cyclodextrins (δ and ε) with 9 and 10 glucose units per macrocycle. In the absence of templates, the transient DCL lasts less than a day, and cyclodextrins convert rapidly to short maltooligosaccharides. Templates stabilize the kinetically trapped subsystem enabling robust selective synthesis of cyclodextrins, as demonstrated by the high-yielding sequential interconversion of cyclodextrins in a single reaction vessel. Our results show that given the right balance between thermodynamic and kinetic control, templates can direct out-of-equilibrium self-assembly, and be used to manipulate enzymatic transformations to favor specific and/or alternative products to those selected in Nature.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...