Dual-wavelength efficient two-photon photorelease of glycine by π-extended dipolar coumarins†
Abstract
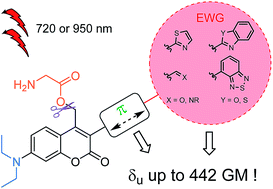
Photolabile protecting groups (PPGs) releasing bioactive compounds upon two-photon excitation have emerged as increasingly popular tools to control and study physiological processes. Yet the limited two-photon photosensitivity of many cages is still a critical issue for applications. We herein report the design, synthesis and photophysical study of polarized extended coumarinyl derivatives which show large two-photon sensitivity (up to 440 GM) at two complementary wavelengths in the NIR spectral range. DFT calculations demonstrate that subtle tuning of polarization in the ground-state and confinement of the photo-induced intramolecular charge transfer upon excitation is responsible for enhancing two-photon absorption while maintaining large uncaging efficiency. These findings open a new engineering route towards efficient coumarinyl PPGs.
- This article is part of the themed collections: Most popular 2019-2020 physical and theoretical chemistry articles and Most popular 2018-2019 physical and theoretical chemistry articles


 Please wait while we load your content...
Please wait while we load your content...