An Al-doped SrTiO3 photocatalyst maintaining sunlight-driven overall water splitting activity for over 1000 h of constant illumination†
Abstract
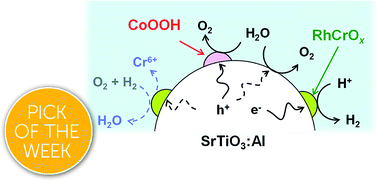
Photocatalytic water splitting is a viable approach to the large-scale production of renewable solar hydrogen. The apparent quantum yield for this reaction has been improved, but the lifespan of photocatalysts functioning under sunlight at ambient pressure have rarely been examined, despite the critical importance of this factor in practical applications. Herein, we show that Al-doped SrTiO3 (SrTiO3:Al) loaded with a RhCrOx (rhodium chromium oxide) cocatalyst splits water with an apparent quantum yield greater than 50% at 365 nm. Moreover, following the photodeposition of CoOOH and TiO2, this material maintains 80% of its initial activity and a solar-to-hydrogen energy conversion efficiency greater than or equal to 0.3% over a span of 1300 h under constant illumination by simulated sunlight at ambient pressure. This result is attributed to reduced dissolution of Cr in the cocatalyst following the oxidative photodeposition of CoOOH. The photodeposition of TiO2 further improves the durability of this photocatalyst. This work demonstrates a concept that could allow the design of long-term, large-scale photocatalyst systems for practical sunlight-driven water splitting.
- This article is part of the themed collections: Editor’s Choice – Jinlong Gong, Most popular 2019-2020 physical and theoretical chemistry articles, Most popular 2018-2019 energy articles, 2019 Chemical Science HOT Article Collection and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...