Monitoring metal–amyloid-β complexation by a FRET-based probe: design, detection, and inhibitor screening†
Abstract
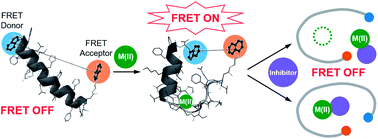
Aggregation of amyloidogenic peptides could cause the onset and progression of neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease. These amyloidogenic peptides can coordinate to metal ions, including Zn(II), which can subsequently affect the peptides' aggregation and toxicity, leading to neurodegeneration. Unfortunately, the detection of metal–amyloidogenic peptide complexation has been very challenging. Herein, we report the development and utilization of a probe (A-1) capable of monitoring metal–amyloid-β (Aβ) complexation based on Förster resonance energy transfer (FRET). Our probe, A-1, is composed of Aβ1–21 grafted with a pair of FRET donor and acceptor capable of providing a FRET signal upon Zn(II) binding even at nanomolar concentrations. The FRET intensity of A-1 increases upon Zn(II) binding and decreases when Zn(II)-bound A-1 aggregates. Moreover, as the FRET intensity of Zn(II)-added A-1 is drastically changed when their interaction is disrupted, A-1 can be used for screening a chemical library to determine effective inhibitors against metal–Aβ interaction. Eight natural products (out of 145 compounds; >80% inhibition) were identified as such inhibitors in vitro, and six of them could reduce Zn(II)–Aβ-induced toxicity in living cells, suggesting structural moieties useful for inhibitor design. Overall, we demonstrate the design of a FRET-based probe for investigating metal–amyloidogenic peptide complexation as well as the feasibility of screening inhibitors against metal-bound amyloidogenic peptides, providing effective and efficient methods for understanding their pathology and finding therapeutic candidates against neurodegenerative disorders.


 Please wait while we load your content...
Please wait while we load your content...