Lytic reactions of drugs with lipid membranes†
Abstract
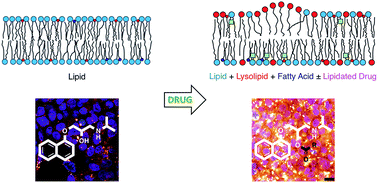
Propranolol is shown to undergo lipidation reactions in three types of lipid membrane: (1) synthetic single-component glycerophospholipid liposomes; (2) liposomes formed from complex lipid mixtures extracted from E. coli or liver cells; and (3) in cellulo in Hep G2 cells. Fourteen different lipidated propranolol homologues were identified in extracts from Hep G2 cells cultured in a medium supplemented with propranolol. This isolation of lipidated drug molecules from liver cells demonstrates a new drug reactivity in living systems. Acyl transfer from lipids to the alcoholic group of propranolol was favoured over transfer to the secondary amine. Migration of acyl groups from the alcohol to the amine was diminished. Other drugs that were examined did not form detectable levels of lipidation products, but many of these drugs did affect the lysolipid levels in model membranes. The propensity for a compound to induce lysolipid formation in a model system was found to be a predictor for phospholipidosis activity in cellulo.
- This article is part of the themed collection: 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...