Investigations on non-classical silylium ions leading to a cyclobutenyl cation†
Abstract
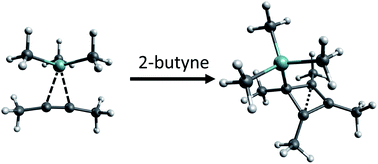
Instead of yielding the desired non-classical silylium ions, the reactions of different alkenes/alkynes with several [Me3Si]+ sources mostly led to oligomerization, or – in the presence of Me3SiH – hydrosilylation of the alkenes/alkynes. Yet, from the reaction of 2-butyne with ion-like Me3Si–F–Al(ORF)3 (RF = C(CF3)3) the salt of the silylated tetramethyl cyclobutenyl cation [Me4C4–SiMe3]+[al–f–al]−1 ([al–f–al]− = [(RFO)3Al–F–Al(ORF)3]−) was obtained in good yield (NMR, scXRD, Raman, and IR). All the experimental and calculated evidence suggest a mechanism in which 1 was formed via a non-classical silylium ion as an intermediate. The removal of the [Me3Si]+ moiety from the cation in 1 was investigated as a means to provide free tetramethyl cyclobutadiene (CBD). However, the addition of [NMe4]F, in order to release Me3SiF and form CBD, led to the unexpected deprotonation of the cation. The addition of 4-dimethylaminopyridine to remove the [Me3Si]+ cation as a Lewis acid/base adduct, led to an adduct with the four-membered ring in the direct neighborhood of the Me3Si group. By the addition of Et2O to a solution of 1, the [F–Al(ORF)3]− anion (and Et2O–Al(ORF)3) was generated from the [al–f–al]− counterion. Subsequently, the [F–Al(ORF)3]− anion abstracted the [Me3Si]+ moiety from [Me4C4–SiMe3]+, probably releasing CBD. However, due to the immediate reaction of CBD with [Me4C4–SiMe3]+ and subsequent oligomerization, it was not possible to use CBD in follow-up chemistry.


 Please wait while we load your content...
Please wait while we load your content...