The inverted ketene synthon: a double umpolung approach to enantioselective β2,3-amino amide synthesis†
Abstract
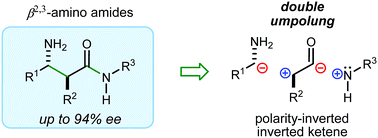
A stereocontrolled synthesis of β2,3-amino amides is reported. Innovation is encapsulated by the first use of nitroalkenes to achieve double umpolung in enantioselective β-amino amide synthesis. Step economy is also fulfilled by the use of Umpolung Amide Synthesis (UmAS) in the second step, delivering the amide product without intermediacy of a carboxylic acid or activated derivative. Molybdenum oxide-mediated hydride reduction provides the anti-β2,3-amino amide with high selectivity.


 Please wait while we load your content...
Please wait while we load your content...