Solid-phase synthesis and structural characterisation of phosphoroselenolate-modified DNA: a backbone analogue which does not impose conformational bias and facilitates SAD X-ray crystallography†
Abstract
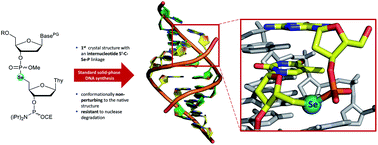
Oligodeoxynucleotides incorporating internucleotide phosphoroselenolate linkages have been prepared under solid-phase synthesis conditions using dimer phosphoramidites. These dimers were constructed following the high yielding Michaelis–Arbuzov (M–A) reaction of nucleoside H-phosphonate derivatives with 5′-deoxythymidine-5′-selenocyanate and subsequent phosphitylation. Efficient coupling of the dimer phosphoramidites to solid-supported substrates was observed under both manual and automated conditions and required only minor modifications to the standard DNA synthesis cycle. In a further demonstration of the utility of M–A chemistry, the support-bound selenonucleoside was reacted with an H-phosphonate and then chain extended using phosphoramidite chemistry. Following initial unmasking of methyl-protected phosphoroselenolate diesters, pure oligodeoxynucleotides were isolated using standard deprotection and purification procedures and subsequently characterised by mass spectrometry and circular dichroism. The CD spectra of both modified and native duplexes derived from self-complementary sequences with A-form, B-form or mixed conformational preferences were essentially superimposable. These sequences were also used to study the effect of the modification upon duplex stability which showed context-dependent destabilisation (−0.4 to −3.1 °C per phosphoroselenolate) when introduced at the 5′-termini of A-form or mixed duplexes or at juxtaposed central loci within a B-form duplex (−1.0 °C per modification). As found with other nucleic acids incorporating selenium, expeditious crystallisation of a modified decanucleotide A-form duplex was observed and the structure solved to a resolution of 1.45 Å. The DNA structure adjacent to the modification was not significantly perturbed. The phosphoroselenolate linkage was found to impart resistance to nuclease activity.


 Please wait while we load your content...
Please wait while we load your content...