Rhodium(i)-catalyzed C6-selective C–H alkenylation and polyenylation of 2-pyridones with alkenyl and conjugated polyenyl carboxylic acids†
Abstract
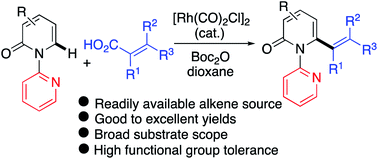
A versatile Rh(I)-catalyzed C6-selective decarbonylative C–H alkenylation of 2-pyridones with readily available, and inexpensive alkenyl carboxylic acids has been developed. This directed dehydrogenative cross-coupling reaction affords 6-alkenylated 2-pyridones that would otherwise be difficult to access using conventional C–H functionalization protocols. The reaction occurs with high efficiency and is tolerant of a broad range of functional groups. A wide scope of alkenyl carboxylic acids, including challenging conjugated polyene carboxylic acids, are amenable to this transformation and no addition of external oxidant is required. Mechanistic studies revealed that (1) Boc2O acts as the activator for the in situ transformation of the carboxylic acids into anhydrides before oxidative addition by the Rh catalyst, (2) a decarbonylation step is involved in the catalytic cycle, and (3) the C–H bond cleavage is likely the turnover-limiting step.


 Please wait while we load your content...
Please wait while we load your content...