Ligand-controlled diastereodivergent, enantio- and regioselective copper-catalyzed hydroxyalkylboration of 1,3-dienes with ketones†
Abstract
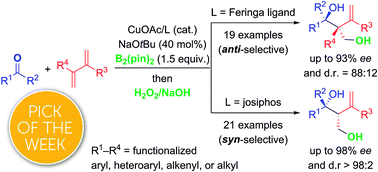
A copper-catalyzed three-component coupling of 1,3-dienes, bis(pinacolato)diboron, and ketones allows for the chemo-, regio-, diastereo- and enantioselective assembly of densely functionalized tertiary homoallylic alcohols. The relative configuration of the vicinal stereocenters is controlled by the chiral ligand employed. Subsequent transformations illustrate the versatility of these valuable chiral building blocks.
- This article is part of the themed collections: 2019 Chemical Science HOT Article Collection and 2019 ChemSci Pick of the Week Collection


 Please wait while we load your content...
Please wait while we load your content...