Low-order many-body interactions determine the local structure of liquid water†
Abstract
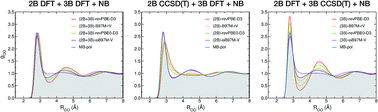
Despite its apparent simplicity, water displays unique behavior across the phase diagram which is strictly related to the ability of the water molecules to form dense, yet dynamic, hydrogen-bond networks that continually fluctuate in time and space. The competition between different local hydrogen-bonding environments has been hypothesized as a possible origin of the anomalous properties of liquid water. Through a systematic application of the many-body expansion of the total energy, we demonstrate that the local structure of liquid water at room temperature is determined by a delicate balance between two-body and three-body energies, which is further modulated by higher-order many-body effects. Besides providing fundamental insights into the structure of liquid water, this analysis also emphasizes that a correct representation of two-body and three-body energies requires sub-chemical accuracy that is nowadays only achieved by many-body models rigorously derived from the many-body expansion of the total energy, which thus hold great promise for shedding light on the molecular origin of the anomalous behavior of liquid water.
- This article is part of the themed collections: Most popular 2019-2020 physical and theoretical chemistry articles and The ChemRxiv Collection


 Please wait while we load your content...
Please wait while we load your content...