Modular supramolecular dimerization of optically tunable extended aryl viologens†
Abstract
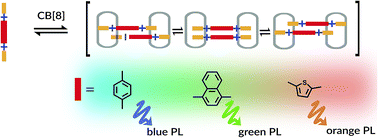
Cucurbit[8]uril (CB[8]) mediated assembly of extended aryl viologens (EVs) into optically tunable dimers is reported for the first time. We show that the modular design and synthesis of a new class of π-conjugated viologen derivatives with rigid aromatic or heteroaromatic bridging units as well as electron donating molecular recognition motifs enable their self-assembly into 2 : 2 complexes with CB[8]. The quantitative dimerization process involving these two molecular components in an aqueous solution enables excimer-like interactions between closely packed charged guests giving rise to distinct spectroscopic behavior. The nature of these dimers (CB[8]2·(EV[X]R)2) in the ground and excited states was characterized by NMR, isothermal titration calorimetry, and steady-state spectroscopic measurements.


 Please wait while we load your content...
Please wait while we load your content...