Combined high degree of carboxylation and electronic conduction in graphene acid sets new limits for metal free catalysis in alcohol oxidation†
Abstract
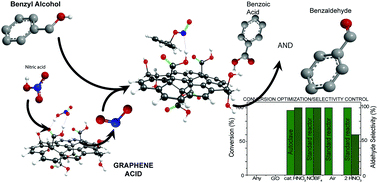
Graphene oxide, the most prominent carbocatalyst for several oxidation reactions, has severe limitations due to the overstoichiometric amounts required to achieve practical conversions. Graphene acid, a well-defined graphene derivative selectively and homogeneously covered by carboxylic groups but maintaining the high electronic conductivity of pristine graphene, sets new activity limits in the selective and general oxidation of a large gamut of alcohols, even working at 5 wt% loading for at least 10 reaction cycles without any influence from metal impurities. According to experimental data and first principles calculations, the selective and dense functionalization with carboxyl groups, combined with excellent electron transfer properties, accounts for the unprecedented catalytic activity of this graphene derivative. Moreover, the controlled structure of graphene acid allows shedding light upon the critical steps of the reaction and regulating precisely its selectivity toward different oxidation products.


 Please wait while we load your content...
Please wait while we load your content...