Gold(i)-catalyzed stereoselective cyclization of 1,3-enyne aldehydes by a 1,3-acyloxy migration/Nazarov cyclization/aldol addition cascade†
Abstract
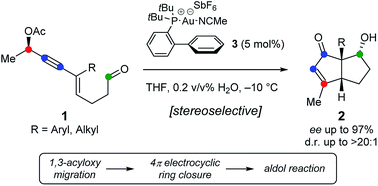
Stereoselective synthesis of bicyclo[3.3.0]octenones from chiral 1,3-enyne aldehydes bearing propargylic acetates is described. The method is based on a Au(I)-catalyzed domino sequence with concomitant transfer of chirality involving 1,3-acyloxy migration followed by Nazarov cyclization and an unprecedented aldol addition. The method furnishes densely functionalized bicyclic structures in high yields, with up to 97% ee and good diastereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...