Low-valent homobimetallic Rh complexes: influence of ligands on the structure and the intramolecular reactivity of Rh–H intermediates†
Abstract
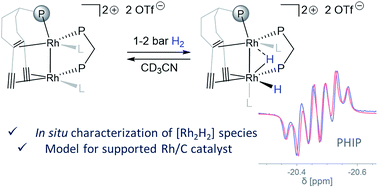
Supporting two metal binding sites by a tailored polydentate trop-based (trop = 5H-dibenzo[a,d]cyclohepten-5-yl) ligand yields highly unsymmetric homobimetallic rhodium(I) complexes. Their reaction with hydrogen rapidly forms Rh hydrides that undergo an intramolecular semihydrogenation of two C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds of the trop ligand. This reaction is chemoselective and converts C
C bonds of the trop ligand. This reaction is chemoselective and converts C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C bonds to a bridging carbene and an olefinic ligand in the first and the second semihydrogenation steps, respectively. Stabilization by a bridging diphosphine ligand allows characterization of a Rh hydride species by advanced NMR techniques and may provide insight into possible elementary steps of H2 activation by interfacial sites of heterogeneous Rh/C catalysts.
C bonds to a bridging carbene and an olefinic ligand in the first and the second semihydrogenation steps, respectively. Stabilization by a bridging diphosphine ligand allows characterization of a Rh hydride species by advanced NMR techniques and may provide insight into possible elementary steps of H2 activation by interfacial sites of heterogeneous Rh/C catalysts.


 Please wait while we load your content...
Please wait while we load your content...