Electrochemical C–H oxygenation and alcohol dehydrogenation involving Fe-oxo species using water as the oxygen source†
Abstract
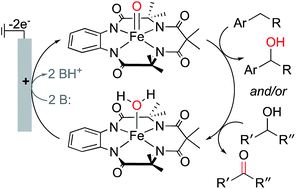
High-valent iron-oxo complexes are key intermediates in C–H functionalization reactions. Herein, we report the generation of a (TAML)Fe-oxo species (TAML = tetraamido macrocyclic ligand) via electrochemical proton-coupled oxidation of the corresponding (TAML)FeIII–OH2 complex. Cyclic voltammetry (CV) and spectroelectrochemical studies are used to elucidate the relevant (TAML)Fe redox processes and determine the predominant (TAML)Fe species present in solution during bulk electrolysis. Evidence for iron(IV) and iron(V) species is presented, and these species are used in the electrochemical oxygenation of benzylic C–H bonds and dehydrogenation of alcohols to ketones.
- This article is part of the themed collections: Sustainable synthesis and catalysis – Chemical Science symposium collection, Most popular 2019-2020 organic chemistry articles and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...