A selective route to aryl-triphosphiranes and their titanocene-induced fragmentation†
Abstract
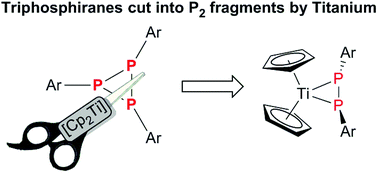
Triphosphiranes are three-membered phosphorus cycles and their fundamental reactivity has been studied in recent decades. We recently developed a high-yielding, selective synthesis for various aryl-substituted triphosphiranes. Variation of the reaction conditions in combination with theoretical studies helped to rationalize the formation of these homoleptic phosphorus ring systems and highly reactive intermediates could be isolated. In addition we showed that a titanocene synthon [Cp2Ti(btmsa)] facilitates the selective conversion of these triphosphiranes into titanocene diphosphene complexes. This unexpected reactivity mode was further studied theoretically and experimental evidence is presented for the proposed reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...