Resolving the structure of the E1 state of Mo nitrogenase through Mo and Fe K-edge EXAFS and QM/MM calculations†
Abstract
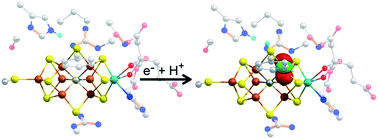
Biological nitrogen fixation is predominately accomplished through Mo nitrogenase, which utilizes a complex MoFe7S9C catalytic cluster to reduce N2 to NH3. This cluster requires the accumulation of three to four reducing equivalents prior to binding N2; however, despite decades of research, the intermediate states formed prior to N2 binding are still poorly understood. Herein, we use Mo and Fe K-edge X-ray absorption spectroscopy and QM/MM calculations to investigate the nature of the E1 state, which is formed following the addition of the first reducing equivalent to Mo nitrogenase. By analyzing the extended X-ray absorption fine structure (EXAFS) region, we provide structural insight into the changes that occur in the metal clusters of the protein when forming the E1 state, and use these metrics to assess a variety of possible models of the E1 state. The combination of our experimental and theoretical results supports that formation of E1 involves an Fe-centered reduction combined with the protonation of a belt-sulfide of the cluster. Hence, these results provide critical experiment and computational insight into the mechanism of this important enzyme.
- This article is part of the themed collection: Most popular 2019-2020 analytical chemistry articles


 Please wait while we load your content...
Please wait while we load your content...