Formamide catalyzed activation of carboxylic acids – versatile and cost-efficient amidation and esterification†
Abstract
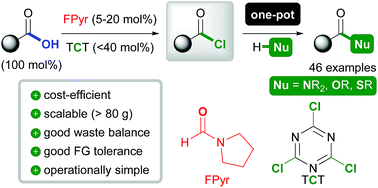
A novel, broadly applicable method for amide C–N and ester C–O bond formation is presented based on formylpyrrolidine (FPyr) as a Lewis base catalyst. Herein, trichlorotriazine (TCT), which is the most cost-efficient reagent for OH-group activation, was employed in amounts of ≤40 mol% with respect to the starting material (100 mol%). The new approach is distinguished by excellent cost-efficiency, waste-balance (E-factor down to 3) and scalability (up to >80 g). Moreover, high levels of functional group compatibility, which includes acid-labile acetals and silyl ethers, are demonstrated and even peptide C–N bonds can be formed. In comparison to reported amidation procedures using TCT, yields are considerably improved (for instance from 26 to 91%) and esterification is facilitated for the first time in synthetically useful yields. These significant enhancements are rationalized by activation by means of acid chlorides instead of less electrophilic acid anhydride intermediates.


 Please wait while we load your content...
Please wait while we load your content...