Antimicrobial silver targets glyceraldehyde-3-phosphate dehydrogenase in glycolysis of E. coli†
Abstract
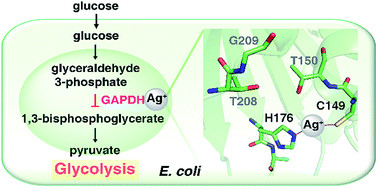
Silver has long been used as an antibacterial agent, yet its molecular targets remain largely unknown. Using a custom-designed coupling of gel electrophoresis with inductively coupled plasma mass spectrometry (GE-ICP-MS), we identified six silver-binding proteins in E. coli. The majority of the identified proteins are associated with the central carbon metabolism of E. coli. Among them, we unveil that GAPDH, an essential enzyme in glycolysis, serves as a vital target of Ag+ in E. coli for the first time. We demonstrate that silver inhibits the enzymatic function of GAPDH through targeting Cys149 in its catalytic site. The X-ray structure reveals that Ag+ coordinates to Cys149 and His176 with a quasi-linear geometry (S–Ag–N angle of 157°). And unexpectedly, two Ag+ ions coordinate to Cys288 in the non-catalytic site with weak argentophilic interaction (Ag⋯Ag distance of 2.9 Å). This is the first report on antimicrobial Ag+ targeting a key enzyme in the glycolytic pathway of E. coli. The findings expand our knowledge on the mode of action and bio-coordination chemistry of silver, particularly silver-targeting residues in proteins at the atomic level.


 Please wait while we load your content...
Please wait while we load your content...