Enantioselective photoredox dehalogenative protonation†
Abstract
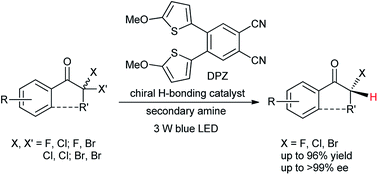
We report an enantioselective photoredox dehalogenative protonation as a new type of asymmetric protonation. As a paradigm, with a cooperative catalytic system consisting of a chiral H-bonding catalyst and a dicyanopyrazine-derived chromophore (DPZ) photosensitizer that is irradiated with visible light, a range of cyclic and acyclic ketones with labile chiral secondary C–F, C–Cl and C–Br bonds at the α-position were obtained in high yields with good to excellent enantioselectivities (up to >99% ee) by using a secondary amine as the terminal reductant. Given the ready accessibility of halides, the success of this work should provide inspiration for constructing diverse chiral α-tertiary carbonyls and their variants.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...