Systematic exploration of the mechanical properties of 13 621 inorganic compounds†
Abstract
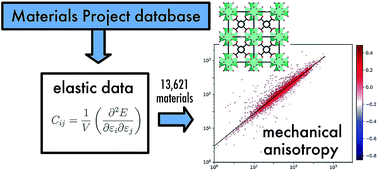
In order to better understand the mechanical properties of crystalline materials, we performed a large-scale exploration of the elastic properties of 13 621 crystals from the Materials Project database, including both experimentally synthesized and hypothetical structures. We studied both their average (isotropic) behavior, as well as the anisotropy of the elastic properties: bulk modulus, shear modulus, Young's modulus, Poisson's ratio, and linear compressibility. We show that general mechanical trends, which hold for isotropic (noncrystalline) materials at the macroscopic scale, also apply “on average” for crystals. Further, we highlight the importance of elastic anisotropy and the role of mechanical stability as playing key roles in the experimental feasibility of hypothetical compounds. We also quantify the frequency of occurrence of rare anomalous mechanical properties: 3% of the crystals feature negative linear compressibility, and only 0.3% have complete auxeticity.
- This article is part of the themed collection: The ChemRxiv Collection


 Please wait while we load your content...
Please wait while we load your content...