Photo-redox reactivity of titanium-oxo clusters: mechanistic insight into a two-electron intramolecular process, and structural characterisation of mixed-valent Ti(iii)/Ti(iv) products†
Abstract
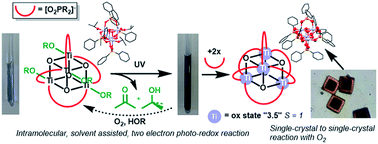
Small titanium-oxo-alkoxide clusters, [TiO(OR)(O2PR′2)]4, synthesised by the stoichiometric reaction of Ti(OiPr)4, phosphinic acid and water, undergo a photo-redox transformation under long-wave UV light. The photo-reaction generates blue coloured, mixed-valence Ti(III)/Ti(IV)-oxo clusters alongside acetone and isopropanol by-products. This reactivity indicates the ability for photoactivated charge separation to occur in even the smallest of Ti-oxo clusters. EPR and NMR spectroscopic studies support a photo-redox mechanism that occurs via an intramolecular, two-electron pathway, directly relating to current doubling effects observed at TiO2 photoanodes in the presence of alcohols. The rate of photo-reaction is solvent dependent, with donor solvents supporting the formation of low coordinate Ti(III) sites. The nature of the electronic transition is identified by DFT and TDDFT calculations as an oxygen to titanium charge transfer and it is possible to finetune the UV absorption onset observed by changing the phosphinate ligand. A two-electron photo-reduced cluster, [Ti4O4(O2PPh2)6], forms spontaneously from the photo-reaction and its structure is identified by X-ray crystallography with supporting DFT calculations. These indicate that [Ti4O4(O2PPh2)6] is high-spin and contains two ferromagnetically coupled electrons delocalised over the Ti4 core. [Ti4O4(O2PPh2)6] undergoes rapid oxidation in air in the solid-state and performs a remarkable single-crystal to single-crystal transformation, to form a stable cluster-superoxide salt.


 Please wait while we load your content...
Please wait while we load your content...