A vinyl silylsilylene and its activation of strong homo- and heteroatomic bonds†
Abstract
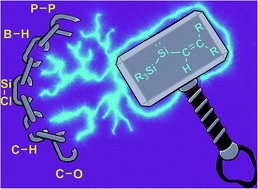
The facile synthesis of a rare two-coordinate acyclic silylene (R2Si:) that is stabilized using a bulky vinylic N-heterocyclic olefin ligand and the strongly σ-donating hypersilyl group [Si(SiMe3)3]− is reported. This vinyl-substituted silylene exhibits an excellent combination of prolonged thermal stability along with high reactivity towards small molecules. Despite being stable for months in solution, the reactivity of this new silylene is manifest in its ambient temperature activation of strong B–H, Si–Cl, C–O, P–P and C–H bonds.


 Please wait while we load your content...
Please wait while we load your content...