NosL is a dedicated copper chaperone for assembly of the CuZ center of nitrous oxide reductase†
Abstract
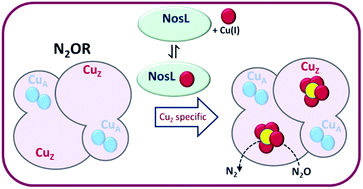
Nitrous oxide reductase (N2OR) is the terminal enzyme of the denitrification pathway of soil bacteria that reduces the greenhouse gas nitrous oxide (N2O) to dinitrogen. In addition to a binuclear CuA site that functions in electron transfer, the active site of N2OR features a unique tetranuclear copper cluster bridged by inorganic sulfide, termed CuZ. In copper-limited environments, N2OR fails to function, resulting in truncation of denitrification and rising levels of N2O released by cells to the atmosphere, presenting a major environmental challenge. Here we report studies of nosL from Paracoccus denitrificans, which is part of the nos gene cluster, and encodes a putative copper binding protein. A Paracoccus denitrificans ΔnosL mutant strain had no denitrification phenotype under copper-sufficient conditions but failed to reduce N2O under copper-limited conditions. N2OR isolated from ΔnosL cells was found to be deficient in copper and to exhibit attenuated activity. UV-visible absorbance spectroscopy revealed that bands due to the CuA center were unaffected, while those corresponding to the CuZ center were significantly reduced in intensity. In vitro studies of a soluble form of NosL without its predicted membrane anchor showed that it binds one Cu(I) ion per protein with attomolar affinity, but does not bind Cu(II). Together, the data demonstrate that NosL is a copper-binding protein specifically required for assembly of the CuZ center of N2OR, and thus represents the first characterised assembly factor for the CuZ active site of this key environmental enzyme, which is globally responsible for the destruction of a potent greenhouse gas.
- This article is part of the themed collections: 2019 Chemical Science HOT Article Collection and Editor's Choice – Serena DeBeer


 Please wait while we load your content...
Please wait while we load your content...