In-depth investigation of large axial magnetic anisotropy in monometallic 3d complexes using frequency domain magnetic resonance and ab initio methods: a study of trigonal bipyramidal Co(ii)†
Abstract
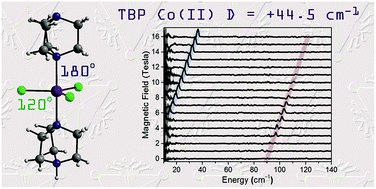
The magnetic properties of 3d monometallic complexes can be tuned through geometric control, owing to their synthetic accessibility and relative structural simplicity. Monodentate ligands offer great potential for fine-tuning the coordination environment to engineer both the axial and rhombic zero-field splitting (ZFS) parameters. In [CoCl3(DABCO)(HDABCO)] (1), the trigonal bipyramidal Co(II) centre has two bulky axial ligands and three equatorial chloride ligands. An in-depth experimental and theoretical study of 1 reveals a large easy-plane magnetic anisotropy (+ve D) with a negligible rhombic zero-field splitting (E) due to the strict axial symmetry imposed by the C3 symmetric ligand and trigonal space group. The large easy-plane magnetic anisotropy (D = +44.5 cm−1) is directly deduced using high-field EPR and frequency-domain magnetic resonance (FDMR) studies. Ab initio calculations reveal a large positive contribution to the D term arising from ground state/excited state mixing of the 4E′′ states at ∼4085 cm−1 and a minor contribution from the spin–flip transition as well. The nature of the slow relaxation in 1 is elucidated through analysis of the rates of relaxation of magnetisation, taking into account Raman and direct spin–lattice relaxation processes and Quantum Tunnelling of the Magnetisation (QTM). The terms relating to the direct process and QTM were found based on the fit of the field-dependence of τ at 2 K. Subsequently, these were used as fixed parameters in the fit of the temperature-dependence of τ to obtain the Raman terms. This experimental–theoretical investigation provides further insight into the power of FDMR and ab initio methods for the thorough investigation of magnetic anisotropy. Thus, these results contribute to design criteria for high magnetic anisotropy systems.


 Please wait while we load your content...
Please wait while we load your content...