Metal vs. ligand protonation and the alleged proton-shuttling role of the azadithiolate ligand in catalytic H2 formation with FeFe hydrogenase model complexes†
Abstract
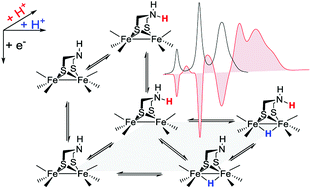
Electron and proton transfer reactions of diiron complexes [Fe2adt(CO)6] (1) and [Fe2adt(CO)4(PMe3)2] (4), with the biomimetic azadithiolate (adt) bridging ligand, have been investigated by real-time IR- and UV-vis-spectroscopic observation to elucidate the role of the adt-N as a potential proton shuttle in catalytic H2 formation. Protonation of the one-electron reduced complex, 1−, occurs on the adt-N yielding 1H and the same species is obtained by one-electron reduction of 1H+. The preference for ligand vs. metal protonation in the Fe2(I,0) state is presumably kinetic but no evidence for tautomerization of 1H to the hydride 1Hy was observed. This shows that the adt ligand does not work as a proton relay in the formation of hydride intermediates in the reduced catalyst. A hydride intermediate 1HHy+ is formed only by protonation of 1H with stronger acid. Adt protonation results in reduction of the catalyst at much less negative potential, but subsequent protonation of the metal centers is not slowed down, as would be expected according to the decrease in basicity. Thus, the adtH+ complex retains a high turnover frequency at the lowered overpotential. Instead of proton shuttling, we propose that this gain in catalytic performance compared to the propyldithiolate analogue might be rationalized in terms of lower reorganization energy for hydride formation with bulk acid upon adt protonation.
- This article is part of the themed collection: Editor's Choice – Serena DeBeer


 Please wait while we load your content...
Please wait while we load your content...