Renewing accessible heptazine chemistry: 2,5,8-tris(3,5-diethyl-pyrazolyl)-heptazine, a new highly soluble heptazine derivative with exchangeable groups, and examples of newly derived heptazines and their physical chemistry†
Abstract
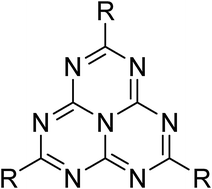
We have prepared 2,5,8-tris(3,5-diethyl-pyrazolyl)-heptazine, the first highly soluble heptazine derivative possessing easily exchangeable leaving groups. We present its original synthesis employing mechanochemistry, along with a few examples of its versatile reactivity. It is, in particular, demonstrated that the pyrazolyl leaving groups can be replaced by several secondary or primary amino substituents or by three aryl- or benzyl-thiol substituents. In addition to being a synthetic platform, 2,5,8-tris(3,5-diethyl-pyrazolyl)-heptazine is fluorescent and electroactive, and its attractive properties, as well as those of the derived heptazines, are briefly presented.


 Please wait while we load your content...
Please wait while we load your content...