Au-catalyzed skeletal rearrangement of O-propargylic oximes via N–O bond cleavage with the aid of a Brønsted base cocatalyst†
Abstract
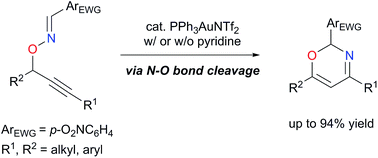
O-Propargylic oximes that possess an electron-withdrawing aryl group on the oxime moiety undergo Au-catalyzed skeletal rearrangements via N–O bond cleavage to afford the corresponding 2H-1,3-oxazine derivatives. Our studies show that the inclusion of a Brønsted base cocatalyst not only accelerates the reaction but also switches pathways of the skeletal rearrangement reaction, realizing divergent synthesis of heterocyclic compounds. Computational studies indicate that the elimination of propargylic proton in the cyclized vinylgold intermediate is rate-determining and both electron-withdrawing substituents at the oxime moiety and base cocatalyst facilitate the proton elimination. Moreover, the protodeauration process proceeds stepwise involving N–O bond cleavage followed by recyclization to construct the oxazine core.


 Please wait while we load your content...
Please wait while we load your content...