The antioxidant activity of polysulfides: it's radical!†
Abstract
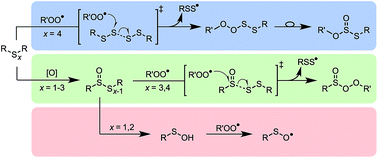
Olefin sulfurization, wherein alkenes and sulfur are heated together at high temperatures, produces branched polysulfides. Due to their anti-wear properties, they are indispensible additives to lubricants, but are also added to other petroleum-derived products as oxidation inhibitors. Polysulfides also figure prominently in the chemistry and biology of garlic and other plants of the Allium species. We previously reported that trisulfides, upon oxidation to their corresponding 1-oxides, are surprisingly effective radical-trapping antioxidants (RTAs) at ambient temperatures. Herein, we show that the homolytic substitution mechanism responsible also operates for tetrasulfides, but not trisulfides, disulfides or sulfides. Moreover, we show that this reactivity persists at elevated temperature (160 °C), enabling tetrasulfides to not only eclipse their 1-oxides as RTAs, but also hindered phenols and alkylated diphenylamines – the most common industrial antioxidant additives. The reactivity is unique to higher polysulfides (n ≥ 4), since homolytic substitution upon them at S2 yields stabilized perthiyl radicals. The persistence of perthiyl radicals also underlies the greater reactivity of polysulfides at elevated temperatures relative to their 1-oxides, since homolytic S–S bond cleavage is reversible in the former, but not in the latter. These results suggest that olefin sulfurization processes optimized for tetrasulfide production will afford materials that impart significantly better oxidation stability to hydrocarbon-based products to which polysulfides are added. Moreover, it suggests that RTA activity may contribute to the biological activity of plant-derived polysulfides.
- This article is part of the themed collection: 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...