Photocatalytic C–H silylation of heteroarenes by using trialkylhydrosilanes†
Abstract
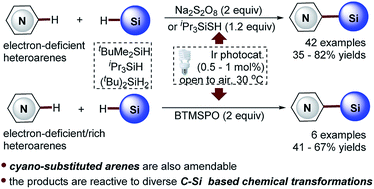
The efficient and selective C–H silylation of heteroarenes, especially the pharmaceutically relevant electron-deficient heteroarenes, represents a great challenge in organic synthesis. Herein we wish to report a distinctive visible light-promoted photocatalytic C–H silylation approach that enables the direct coupling of trialkylhydrosilanes with both electron-deficient and -rich heteroarenes as well as with cyano-substituted arenes in moderate to high yields and with good regioselectivity. The protocol features operational simplicity, mild reaction conditions, and the use of safe and readily available Na2S2O8, bis(trimethylsilyl) peroxide (BTMSPO) or iPr3SiSH as the radical initiators. Notably, the challenging bulky and inert trialkylhydrosilanes, such as (t-butyldimethyl)silane (tBuMe2SiH) and (triisopropyl)silane (iPr3SiH), work smoothly with the protocol. Moreover, despite the higher stability of tBuMe2Si silylation products, our studies revealed their great reactivity and versatility in diverse C–Si-based chemical transformations, providing an operationally simple, low-cost, and environmentally benign synthetic technology for molecule construction and elaboration.


 Please wait while we load your content...
Please wait while we load your content...