Vanadium(V) oxide clusters synthesized by sublimation from bulk under fully inert conditions†
Abstract
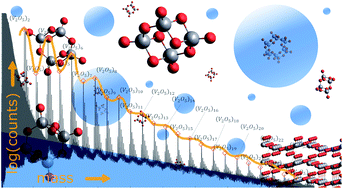
Oxide nanoparticles in the size range of a few nanometers are typically synthesized in solution or via laser ablation techniques, which open numerous channels for structural change via chemical reactions or fragmentation processes. In this work, neutral vanadium oxide nanoparticles are instead synthesized by sublimation from bulk in combination with a pickup by superfluid helium droplets. Mass spectroscopy measurements clearly demonstrate the preservation of the bulk stoichiometric ratio of vanadium to oxygen in He-grown nanoparticles, indicating a tendency towards tetrahedral coordination of the vanadium centers in finite geometries. This unexpected finding opens up new possibilities for a combined on-the-fly synthesis of nanoparticles consisting of metal and metal-oxide layers. In comparison to mass spectra obtained via direct ionization of vanadium oxide in an effusive beam, where strong fragmentation occurred, we observe a clear preference for (V2O5)n oligomers with even n inside the He nanodroplets, which is further investigated and explained using the electronic structure theory.


 Please wait while we load your content...
Please wait while we load your content...