Wave-shaped polycyclic hydrocarbons with controlled aromaticity†
Abstract
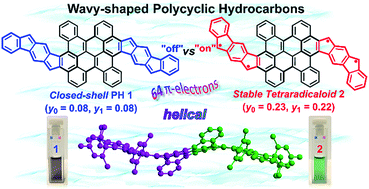
Controlling the aromaticity and electronic properties of curved π-conjugated systems has been increasingly attractive for the development of novel functional materials for organic electronics. Herein, we demonstrate an efficient synthesis of two novel wave-shaped polycyclic hydrocarbons (PHs) 1 and 2 with 64 π-electrons. Among them, the wave-shaped π-conjugated carbon skeleton of 2 is unambiguously revealed by single-crystal X-ray crystallography analysis. The wave-shaped geometry is induced by steric congestion in the cove and fjord regions. Remarkably, the aromaticity of these two structural isomers can be tailored by the annulated direction of cyclopenta[b]fluorene units. Isomer 1 (Eoptg = 1.13 eV) behaves as a closed-shell compound with weakly antiaromatic feature, whereas its structural isomer 2 displays a highly stable tetraradical character (y0 = 0.23; y1 = 0.22; t1/2 = 91 days) with a narrow optical energy gap of 0.96 eV. Moreover, the curved PH 2 exhibits remarkable ambipolar charge transport in solution-processed organic thin-film transistors. Our research provides a new insight into the design and synthesis of stable functional curved aromatics with multiradical characters.
- This article is part of the themed collection: Functional Organic Materials Symposium Collection


 Please wait while we load your content...
Please wait while we load your content...