Catalytic radical difluoromethoxylation of arenes and heteroarenes†
Abstract
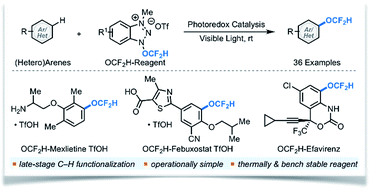
Intermolecular C–H difluoromethoxylation of (hetero)arenes remains a long-standing and unsolved problem in organic synthesis. Herein, we report the first catalytic protocol employing a redox-active difluoromethoxylating reagent 1a and photoredox catalysts for the direct C–H difluoromethoxylation of (hetero)arenes. Our approach is operationally simple, proceeds at room temperature, and uses bench-stable reagents. Its synthetic utility is highlighted by mild reaction conditions that tolerate a wide variety of functional groups and biorelevant molecules. Experimental and computational studies suggest single electron transfer (SET) from excited photoredox catalysts to 1a forming a neutral radical intermediate that liberates the OCF2H radical exclusively. Addition of this radical to (hetero)arenes gives difluoromethoxylated cyclohexadienyl radicals that are oxidized and deprotonated to afford the products of difluoromethoxylation.
- This article is part of the themed collections: 2019 Chemical Science HOT Article Collection and Editor's choice - Paolo Melchiorre


 Please wait while we load your content...
Please wait while we load your content...