Iron sulfur clusters in protein nanocages for photocatalytic hydrogen generation in acidic aqueous solutions†
Abstract
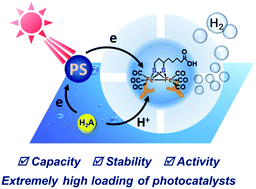
We took advantage of the iron binding affinity of apoferritin to immobilize iron–sulfur clusters into apoferritin up to 312 moieties per protein, with a loading rate as high as 25 wt%. The photocatalytic hydrogen generation activity in acidic aqueous solutions was achieved with TONs up to 31 (based on a single catalyst moiety) or 8.3 × 103 (based on a single protein) upon 3 h of visible light irradiation. The present study provides a versatile strategy to construct uniform protein/photocatalyst supramolecular systems with FeFe-H2ase activity.
- This article is part of the themed collection: Celebrating a century of chemical excellence at Nanjing University


 Please wait while we load your content...
Please wait while we load your content...