Pd-catalyzed γ-arylation of γ,δ-unsaturated O-carbamates via an unusual haptotropic rearrangement†
Abstract
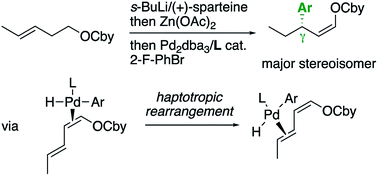
An unusual γ-selectivity was observed in the arylation of γ,δ-unsaturated O-carbamates involving directed lithiation, transmetallation to zinc and Negishi coupling, when a specific combination of aryl electrophile and phosphine ligand is employed. Mechanistic studies indicate that an unusual, stereospecific haptotropic rearrangement of the palladium–diene intermediate is involved.


 Please wait while we load your content...
Please wait while we load your content...