Rare “Janus”-faced
 single-molecule magnet exhibiting intramolecular ferromagnetic interactions†
single-molecule magnet exhibiting intramolecular ferromagnetic interactions†
Abstract
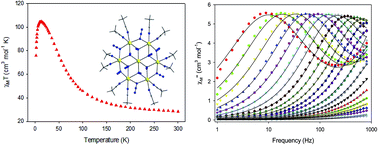
A rare 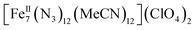 disk-like single-molecule magnet (SMM) exclusively bridged by end-on azides with a spin ground state of S = 14 was prepared by the reaction of a divalent FeII precursor with Me3SiN3 under basic conditions. AC magnetic susceptibility studies revealed unusual, “Janus”-faced SMM behavior for the dried and pristine forms of the
disk-like single-molecule magnet (SMM) exclusively bridged by end-on azides with a spin ground state of S = 14 was prepared by the reaction of a divalent FeII precursor with Me3SiN3 under basic conditions. AC magnetic susceptibility studies revealed unusual, “Janus”-faced SMM behavior for the dried and pristine forms of the 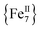 compound attributed to solvation/de-solvation effects of the coordinated MeCN ligands which leads to alterations in the crystal field and symmetry of the metal ions. DFT calculations confirmed the ferromagnetic nature of the interactions between the FeII spin carriers with the zero-field splitting parameters D = −0.2323 cm−1 and E/D = 0.027. The results have important implications for the future study of single-molecule magnets incorporating volatile solvent molecules in the first coordination sphere of the metal ions and their effect on the relaxation dynamics.
compound attributed to solvation/de-solvation effects of the coordinated MeCN ligands which leads to alterations in the crystal field and symmetry of the metal ions. DFT calculations confirmed the ferromagnetic nature of the interactions between the FeII spin carriers with the zero-field splitting parameters D = −0.2323 cm−1 and E/D = 0.027. The results have important implications for the future study of single-molecule magnets incorporating volatile solvent molecules in the first coordination sphere of the metal ions and their effect on the relaxation dynamics.
- This article is part of the themed collections: Most popular 2019-2020 inorganic, main group and crystal engineering chemistry articles and 2018 Chemical Science HOT Article Collection



 Please wait while we load your content...
Please wait while we load your content...
 single-molecule magnet exhibiting intramolecular ferromagnetic interactions
single-molecule magnet exhibiting intramolecular ferromagnetic interactions