Reversibility and reactivity in an acid catalyzed cyclocondensation to give furanochromanes – a reaction at the ‘oxonium-Prins’ vs. ‘ortho-quinone methide cycloaddition’ mechanistic nexus†
Abstract
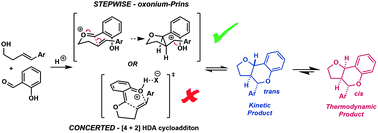
Herein we report a combined experimental and computational investigation of the acid catalyzed cyclocondensation reaction between styrenyl homoallylic alcohols and salicylaldehyde to form furanochromanes. We disclose a previously unreported isomerisation of the ‘unnatural’ trans-fused products to the diastereomeric ‘natural’ cis-fused congeners. Notwithstanding the appeal of assuming this corresponds to endo to exo isomerisation of Diels–Alder (D–A) adducts via concerted retro-cycloaddition/cycloaddition reactions of an in situ generated ortho-quinone methide with the styrenyl alkene, our combined Hammett/DFT study reveals a stepwise Prins-like process via discrete benzylic carbocation intermediates for all but the most electron deficient styrenes. As these reactions fortuitously lie at the intersection of these two mechanistic manifolds, it allows us to propose an experimentally determined indicative ρ+ value of ca. −3 as marking this nexus between a stepwise Prins-type pathway and a concerted cycloaddition reaction. This value should prove useful for categorising other reactions formally involving ‘ortho-quinomethides’, without the need for the extensive computation performed here. Logical optimisation of the reaction based upon the mechanistic insight led to the use of HFIP as an additive which enables exclusive formation of ‘natural’ cis-fused products with a ∼100-fold reaction rate increase and improved scope.
- This article is part of the themed collections: Celebrating our 2020 Prize and Award winners and 2018 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...