Mechanistic investigation into the C(sp3)–H acetoxylation of morpholinones†
Abstract
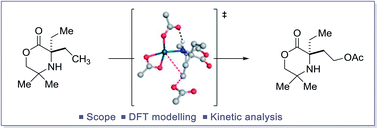
The study of a selective palladium(II)-catalyzed C(sp3)–H acetoxylation reaction on a class of cyclic alkyl amines is reported. Computational modelling and kinetic studies were used to provide support for a mechanism involving selective C–O bond formation from a γ-aminoalkyl-Pd(IV) intermediate. The C–O bond forming step was computed to occur by a dissociative ionization mechanism followed by an SN2 process involving external acetate attack at the C–Pd(IV) bond. This pathway was computed to be of lowest energy with no competing C–N products observed. Additionally, with a few modifications to reaction conditions, preliminary studies showed that this process could be rendered enantioselective in the presence of a non-racemic BINOL-phosphoric acid.
- This article is part of the themed collections: Sustainable synthesis and catalysis – Chemical Science symposium collection and 2019 Chemical Science HOT Article Collection


 Please wait while we load your content...
Please wait while we load your content...