Facile controlled synthesis of Ag3PO4 with various morphologies for enhanced photocatalytic oxygen evolution from water splitting†
Abstract
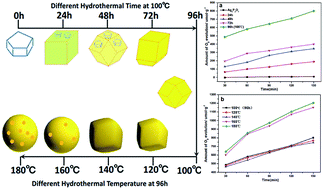
A facile and green hydrothermal method has been developed for the synthesis of Ag3PO4 with a variety of morphologies, including cubic, rhombic dodecahedral, spherical and roughly spherical, by using Ag4P2O7 as a sacrificial precursor. The as-prepared catalysts were characterized by carrying out X-ray diffraction (XRD), scanning electron microscopy (SEM), UV-visible diffuse reflectance spectroscopy (UV-Vis DRS), Fourier transform infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS). The morphology of Ag3PO4 was controlled by simply adjusting the hydrothermal reaction temperature and time, without adding any templates and organic additives. Kinetics studies and characterization results revealed that the transformation from P2O74− to a PO43− radical was a rate-determining step, and influenced the morphology of Ag3PO4. Different oxygen evolution rates were observed for samples subjected to different hydrothermal reaction times, and the highest initial rate of O2 evolution achieved was 582.55 μmol h−1 g−1. Furthermore, for the samples prepared using a hydrothermal reaction time of 96 h, as the hydrothermal reaction temperature was increased, the oxygen evolution rate of the resulting sample decreased first and then increased, and the highest initial rate of O2 evolution was 856.06 μmol h−1 g−1, about twice the 418.34 μmol h−1 g−1 value for the sample prepared using the coprecipitation method. A possible mechanism has been proposed to explain how the hydrothermal reaction temperature and time influenced the Ag3PO4 morphology. Our method provides a guiding hydrothermal strategy for the synthesis of insoluble electrolytes with various morphologies from relatively soluble electrolytes without the need to use templates and organic additives.


 Please wait while we load your content...
Please wait while we load your content...