Structure evolution of chromium-doped boron clusters: toward the formation of endohedral boron cages†
Abstract
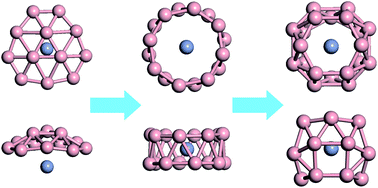
The electron-deficient nature of boron endows isolated boron clusters with a variety of interesting structural and bonding properties that can be further enriched through metal doping. In the current work, we report the structural and electronic properties of a series of chromium-doped boron clusters. The global minimum structures for CrBn clusters with an even number of n ranging from 8 to 22 are proposed through extensive first-principles swarm-intelligence structure searches. Half-sandwich structures are found to be preferred for CrB8, CrB10, CrB12 and CrB14 clusters and to transform to a drum-like structure at CrB16 cluster. Endohedral cage structures with the Cr atom located at the center are energetically most favorable for CrB20 and CrB22 clusters. Notably, the endohedral CrB20 cage has a high symmetry of D2d and a large HOMO–LUMO gap of 4.38 eV, whose stability is attributed to geometric fit and formation of an 18-electron closed-shell configuration. The current results advance our understanding of the structure and bonding of metal-doped boron clusters.


 Please wait while we load your content...
Please wait while we load your content...