Total synthesis of squafosacin F: stereodivergent approach to mono-tetrahydrofuran acetogenins†
Abstract
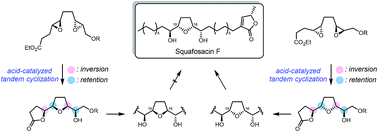
Annonaceous acetogenins have a wide range of potential biological activities. The development of simple and diversity-oriented approaches to their synthesis is therefore important. We have achieved the first total synthesis of squafosacin F and assigned its absolute configuration. The key steps were an acid-mediated tandem intramolecular double cyclization to build the hydroxy-flanked mono-tetrahydrofuran core and decoration with the desired functionalities of the target natural product via highly stereoselective reactions.


 Please wait while we load your content...
Please wait while we load your content...