Copper chloride as a conversion-type positive electrode for rechargeable aluminum batteries
Abstract
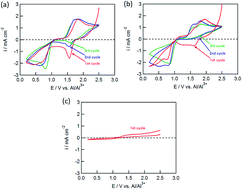
Copper chloride (CuCl2) was investigated for the first time as conversion-type positive electrode material in a rechargeable Al battery. The electrode was reversibly charged and discharged in an electrolyte solution of AlCl3, dipropylsulfone, and toluene (1 : 10 : 5 molar ratio). The initial discharge capacity was about 370 mA h (g-CuCl2)−1 at 0.028C-rate (11 mA (g-CuCl2)−1), which was almost the same as the theoretical value (399 mA h (g-CuCl2)−1) and higher than that of insertion-type positive electrode materials as used in the rechargeable Al battery. Moreover, a two-stage discharge plateau voltage was observed at 1.5 V and 0.8 V, which was higher than other conversion type positive electrodes for the aluminum rechargeable battery. The high discharge voltage realized a high energy density of 426 mW h (g-CuCl2)−1, which is the highest energy density compared with other conversion type positive electrodes. Two different strategies were implemented to increase the lifetime of the cell, namely, increasing the upper cut-off voltage and decreasing the particle size of CuCl2. The discharge capacity for the electrode at the second cycle was threefold that for a pristine CuCl2 electrode.


 Please wait while we load your content...
Please wait while we load your content...